How Does a Car Battery Work?
Ever wondered what exactly happens when you turn the key or press the start button of your car? In just a split second, a key component of your car that brings the engine to life. The heart of this process depends on the car battery. It plays a crucial role in starting the engine. But how can a box, simply filled with acid and metal plates, generate the energy needed to get your engine running? In this article, we will explore these questions and uncover the answers.
A Typical Car Battery Working Mechanism
After looking at this step-by-step process, the battery’s purpose becomes clearer: to supply sufficient power to run the starter motor which is meshed with the flywheel of the engine.
In the battery, this energy is stored in a chemical form, which then is converted into electrical power, delivering a high current to run the starter motor. The starter motor relies on the battery to function, and so does the engine. The crankshaft of the engine begins to turn and starts the combustion engine. This process consumes a lot of energy taken by the battery; therefore, the energy must be restored. The engine connects and rotates the alternator. As the engine runs, the alternator rotates to produce electricity, which is fed back to the battery to recharge it. In other words, electrical energy is converted into chemical energy for storage, as electrical energy cannot be stored directly, but energy can exist in different forms.
Now, let’s take a closer look at how these energy transformations happen.
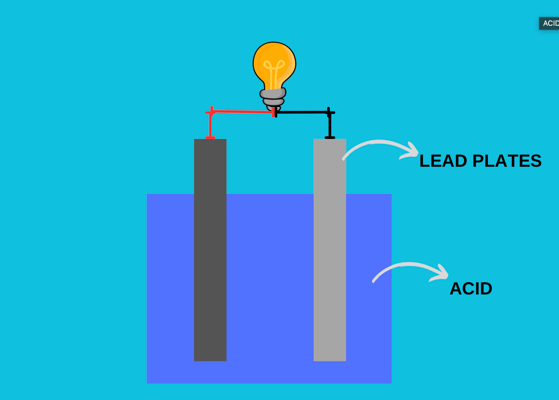
A typical car battery is called a lead-acid battery, as inside the battery, the lead plates are submerged into an acid that releases electrons through chemical reactions. Take the battery; it is surrounded by a plastic cap and a lid holding all internal components. When you remove the lid, you will see six different cells, each generating 2.1 V. Since these cells are connected in series, it makes 12.6 V in total.
Two plates inside the acid consist of dissimilar materials, positive (cathode) and negative (anode) terminals, which form a chemical reaction and release electrons. The cathode is made of lead oxide, whereas the anode is made of pure lead. The acid is a mixture of sulfuric acid and water, which is known as an electrolyte.
The positive terminal reacts with the sulfate in the electrolyte, and the outcome is an oxygen ion ejected from the cathode into the electrolyte. This oxygen ion combines with the hydrogen ions to form water. At the same time, the lead atoms on the anode react with the sulfate ions in the electrolyte. After these reactions, 2 electrons are released and collected in the negative terminal. Now, imagine numerous atoms reacting with each other, releasing countless electrons to be collected in the negative terminal. Since electrons are negatively charged, there is a difference in charge across two terminals. You measure this difference with a voltmeter and see it is 2.1 V. But don’t forget; there are six cells connected in series at the battery that makes 12.6 V in total. So, this is how you can convert electrical power from chemical energy.
However, this reaction doesn’t last forever, after some time, the chemicals required to form a chemical reaction will run out, and the acid becomes weaker. For that reason, the reaction must be reversed. This is the part where the alternator becomes essential. The reaction can be reversed if the battery is supplied with electricity from the alternator. The electrons return to the negative terminal, causing the exact opposite of the reactions described above. For example, the oxygen atoms were removed from the positive plate, but at this time, oxygen will be ejected from the electrolyte and rejoined to the positive (cathode) terminal.
How Does a Typical Car Battery Finish?
A typical car battery loses its function due to natural causes and improper usage.
If the battery is left fully discharged, the sulfate layer separates from the electrolyte and accumulates at the bottom; these sulfates are not possible to be used again, even if the battery is charged. Now, imagine the battery is not left fully discharged for too long but is regularly used and charged many times. Even then, the sulfate layer gradually separates little by little. Over time, typically after about 4 years, the effects become more noticeable. This problem is known as sulfation.
Moreover, every time the battery charges and discharges, the active materials on the plates undergo stress. And over thousands of cycles, these materials become less effective causing capacity to decrease.
The other problem is the corrosion of internal plates. As time passes, the chemical reactions inside the battery cause the lead plates to corrode. If lead plates corrode, active materials decrease and weaken the battery’s structure.
Finally, the last reason is caused by the driver’s carelessness. As it is said before, the alternator provides the power for electrical systems such as headlights. But, when the car is off, the alternator doesn’t work anymore, the power instead is taken by the battery itself which causes the battery to finish very quickly. Therefore, when the engine is off, it’s best to turn off the electrical systems and leave it unused.
In conclusion, the battery of the car is a powerful component to start the engine by providing sufficient electrical power to the starter motor. Then, the starter motor disengages from the engine after the engine runs independently. A smooth ignition process is possible as long as the battery is in good condition. The battery must be charged by the alternator to be used again. During these processes, energy transformation plays a crucial role in the battery. For instance, chemical energy is converted into electrical power to run the engine. And exactly the opposite happens when the battery is charged. However, like any other component, a car battery has a limited life span due to sulfation, corrosion, and improper usage. With proper care and maintenance of the battery, such as avoiding deep discharge and minimizing power usage after the engine is off, the driver can maximize the efficiency of the battery. In the end, a battery in good condition is one of the key points to having a driving experience without trouble.
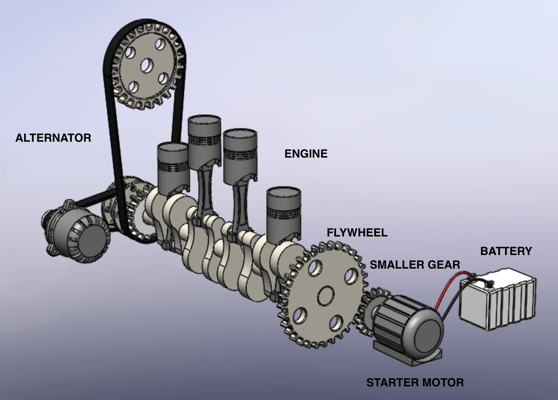
A Starting Car Mechanism
Lead Plates and Acid of a Battery
Eren Erden
Get in Touch
02erenerden@gmail.com
